In chemistry, many equations need to be balanced to get how chemicals will combine or react with one another. A balancing equations worksheet is a presentation which presents chemical equations and clearly states how many atoms of a compound react with another to give an end result of the compounds. We provide you with a useful balancing equations worksheet template which you can use to structure your work appropriately when presenting it. The model will substantially boost your grade when combined with your final test scores. You May also See Algebraic Subtraction Worksheets.
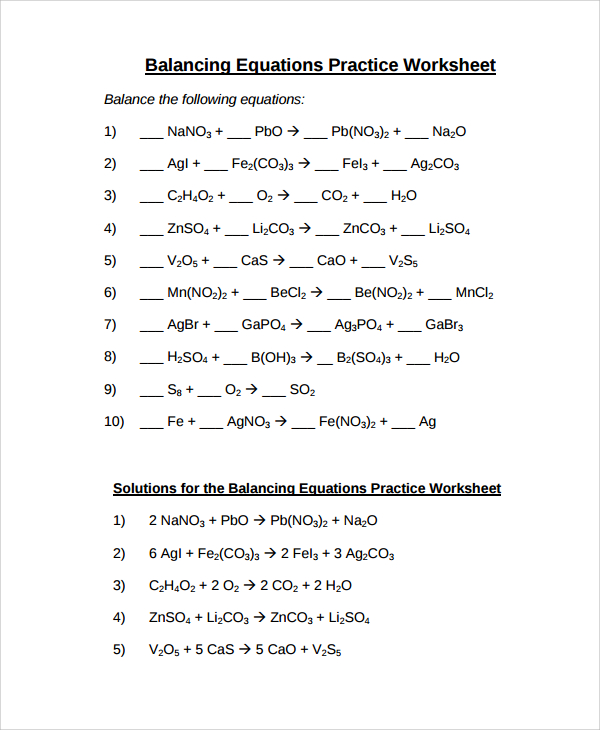
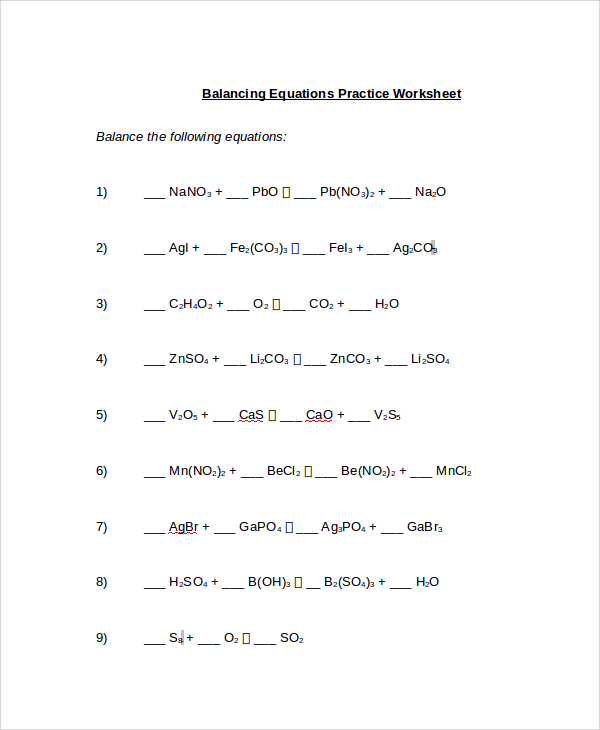
Balancing Equations Practice Worksheet
The model has two pages whereby the first begins with a heading at the top indicating that it is a balancing equations worksheet for identification. The questions of the equations that need to be balanced are all listed putting dashes before every compound in the question. You already know that balancing involves determining how many atoms of a substance react with another to form other elements.
Finally, the second page requires you to put down all the equations that need to be balanced again, although, this time balancing them without putting dashes like on the initial page. The heading of the working should be “solution for the balancing equations worksheet.
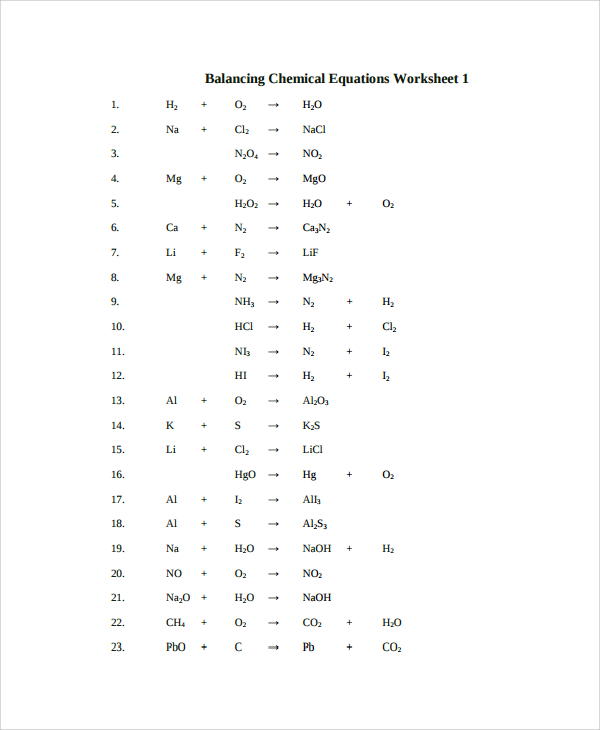
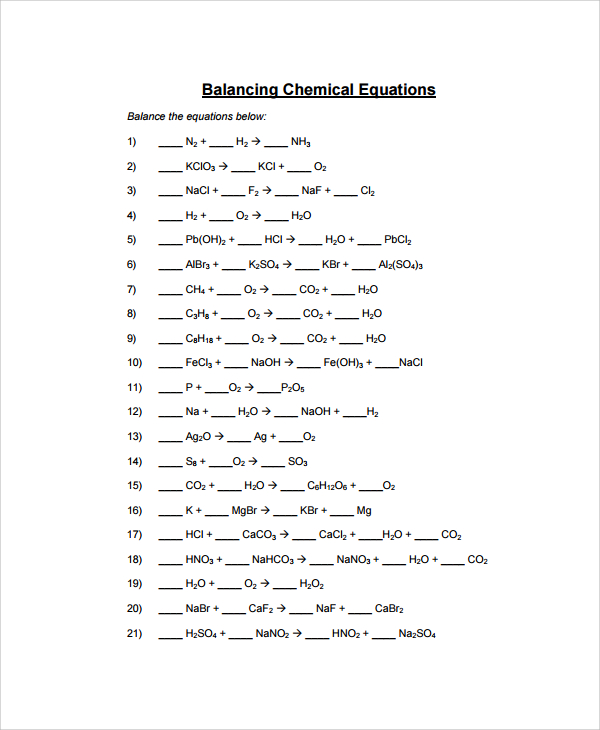
Balancing Chemical Equations Worksheet
The model is designed to cover more work than the previous one. The first page of the template requires you to indicate that the page is the first worksheet and below list all the equations leaving spaces to put the balancing figures or atoms. Since the equations are many, those that will have to spill over to the second page should be numbered as a continuation of those on the previous page.
Also, the top of the second page should state that it is the second worksheet. After listing all the balancing equations questions, on the third page, the top should read that below are answers for the first worksheet before proving the worked out balanced equations. Finally, for those equations that spilled over to the second sheet, put down their workings on a fourth page and at the top notify that the answers are for the second worksheet.
Why is it Important to Balance the Chemical Equations?
Balancing chemical equations is essential for several reasons, all of which underscore its fundamental role in chemistry and related fields:
- Conservation of Mass: The law of conservation of mass states that mass cannot be created or destroyed in an ordinary chemical reaction. Balancing equations ensures that the number of atoms for each element is the same on both sides of the equation, thereby adhering to this law.
- Accurate Representation of Chemical Reactions: A balanced equation accurately represents the actual reaction occurring at the molecular level. It provides a precise description of the reactants involved and the products formed.
- Stoichiometry: Balancing chemical equations is crucial for stoichiometry, which involves calculating the relative quantities of reactants and products in chemical reactions. Accurate stoichiometry is essential for preparing solutions, predicting yields, and scaling up reactions from the laboratory to industrial production.
- Safety and Efficiency: In industrial settings and laboratories, balanced equations help ensure the safety and efficiency of chemical processes. Knowing the exact proportions of reactants prevents the wasteful excess of one reactant and the dangerous shortage of another.
- Educational and Research Purposes: For students and researchers, balanced equations are fundamental tools. They facilitate a deeper understanding of chemical processes and are essential for experimental work and report writing.
- Quantitative Analysis: In chemical manufacturing and engineering, balanced equations are used to calculate the optimal conditions needed for a reaction, such as temperature and pressure, and to maximize the conversion of reactants to products.
- Environmental Impact: Properly balanced equations help in assessing the environmental impact of a reaction. This includes understanding the types and amounts of waste produced, which aids in developing strategies for waste management and reduction.
In summary, balancing chemical equations is vital for theoretical and practical applications in chemistry, ensuring that chemical reactions are understood, executed safely, effectively managed, and environmentally responsible.
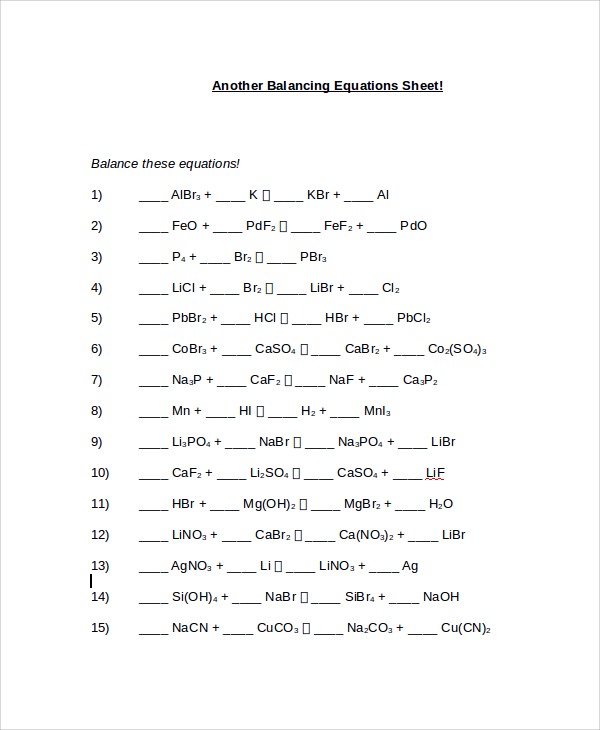
Easy Balancing Equations Worksheet
The model is meant to tackle few additional equations, therefore; it can just be two pages. The heading of the paper should read that this is another balancing equations sheet sample different from a previous one. Then, proceed with listing the balancing equation questions. The second page should indicate the answers to the previous question on the first page. Finally, when putting the missing data that was required in the balancing equation questions ensure that they are in bold for the tutor to distinguish them.
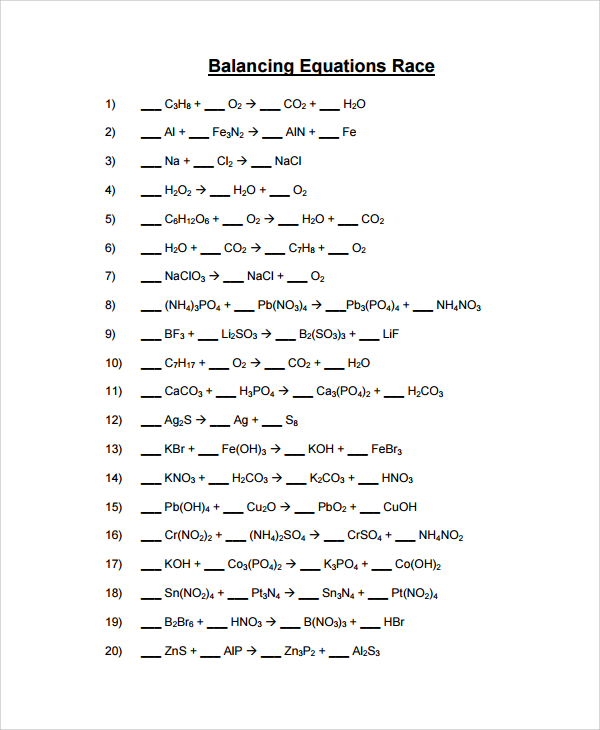
Balancing Equations Race Worksheet
The heading of the model should be “balancing equations race” and the proceeds on with listing the balancing equations questions putting spaces at every place you will need to put your answers. Then, the second page should indicate the solutions to the “balancing equations race.” When listing the answers, in the points where you had let dashes those text you now fill in should be in red to distinct them from the original question.
Advantages of having a Balancing Equation Worksheet Template Since academics are very tiring, the document’s design is ready for you, therefore, reducing for the task of constructing one. The template also guides you structure your work to make it look presentable for your tutor. Finally, since the document is a file you can save on your computer, you are at liberty to make other copies for use in other assignments.
What are Different Types of Equations?
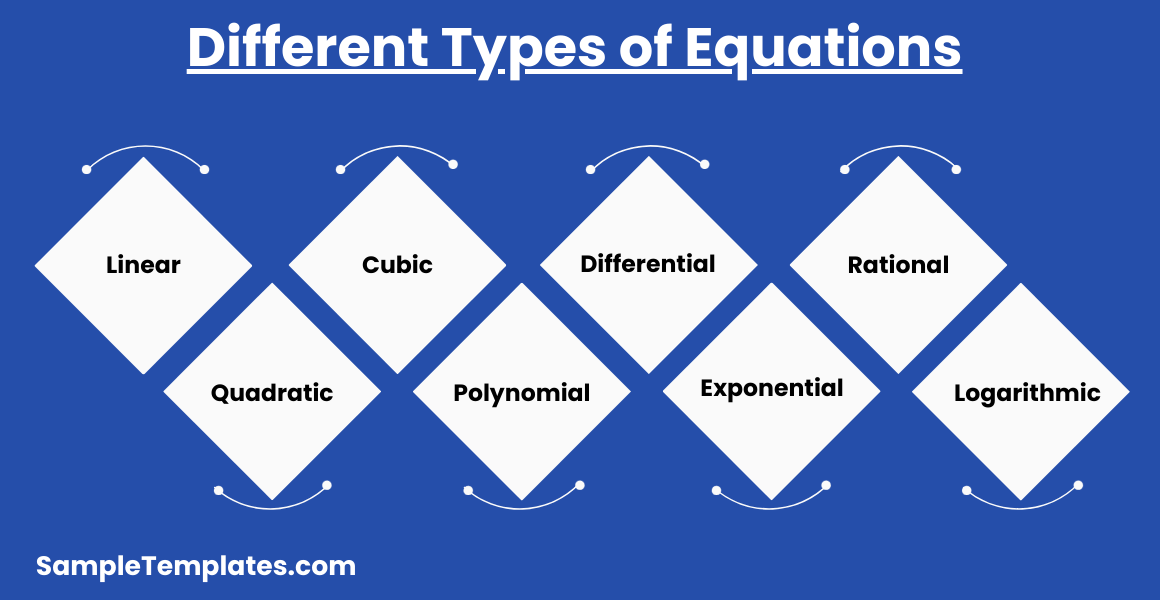
Equations are mathematical statements that assert the equality of two expressions. They play a crucial role in a wide range of disciplines, from basic arithmetic to advanced calculus and engineering. Here are some common types of equations:
- Linear Equations:
- These are first-order equations involving no higher powers than the first power of the variable. They have the general form ??+?=?, where ?, ?, and ? are constants, and ? is the variable.
- Quadratic Equations:
- Quadratic equations involve the second power of the variable (but no higher powers), typically expressed in the form ??2+??+?=0. They can have two, one, or no real solutions depending on the discriminant (?2?4??).
- Cubic Equations:
- These involve the third power of the variable and have the form ??3+??2+??+?=0. Solving cubic equations can be more complex and often requires more advanced algebraic techniques or numerical methods.
- Polynomial Equations:
- A polynomial equation involves a polynomial expression of degree ? set equal to zero, ????+???1???1+…+?2?2+?1?+?0=0. The complexity of solving increases with the degree of the polynomial.
- Differential Equations:
- These involve functions and their derivatives. Differential equations are fundamental in describing various physical phenomena such as motion, heat, and growth. They can be classified further into ordinary differential equations (ODEs) and partial differential equations (PDEs).
- Exponential Equations:
- These equations involve exponential expressions, typically of the form ????=?, where ? is the base of the natural logarithm, and ?, ?, and ? are constants. Solving them often involves logarithmic functions.
- Rational Equations:
- These contain ratios of polynomial expressions. An example is ??+?=?. Special attention is needed to address potential undefined points (where the denominator equals zero).
- Trigonometric Equations:
- These equations involve trigonometric functions. An example is sin?(?)=0.5. Solutions to trigonometric equations often require knowledge of the periodic nature of these functions.
- Absolute Value Equations:
- These involve expressions within absolute value signs, such as ???+??=?. Solving these equations typically involves considering both the positive and negative scenarios of the expression inside the absolute value.
- Logarithmic Equations:
- These involve logarithmic functions and have forms such as log??(?)=?. Solving logarithmic equations requires understanding logarithmic identities and properties.
Each type of equation has its own set of characteristics and methods for finding solutions, making them suited to different applications and fields of study.
Stoichiometry Balancing Equations Worksheet
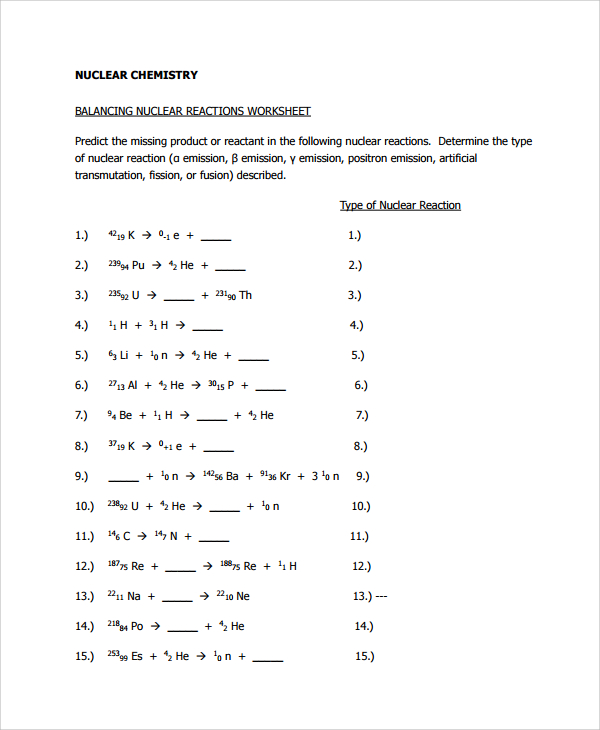
Balancing Nuclear Equations Worksheet
Methods You Can Use For Balancing Equation
Balancing chemical equations is essential for representing chemical reactions accurately. There are several methods you can use to achieve this, each with its own advantages. Here are some commonly used methods:
- Inspection or Trial and Error Method:
- This is the most straightforward method and involves adjusting the coefficients of reactants and products until the number of atoms for each element is the same on both sides of the equation.
- It works well for simpler equations and is often the first method taught.
- Algebraic Method:
- In this method, you assign algebraic variables to the coefficients of the compounds in the unbalanced equation and set up equations based on the conservation of each element.
- Solve the set of simultaneous equations to find the coefficients that balance the equation. This method is more systematic and useful for complex equations.
- Redox Method:
- This approach is particularly useful for balancing redox (oxidation-reduction) reactions. It involves separating the reaction into two half-reactions, one for oxidation and one for reduction.
- Balance the atoms and charges in each half-reaction independently by adding electrons, water, or hydrogen ions as needed. Then, combine the half-reactions, ensuring that the electrons are balanced.
- Matrix Method (using linear algebra):
- For those comfortable with higher mathematics, this method involves setting up a matrix that represents the stoichiometry of the reaction. You then use techniques from linear algebra to solve for the coefficients.
- This is particularly useful for very complex reactions and can be handled using computer software.
- Ion-Electron Method:
- Similar to the Redox method, this technique is used for reactions in an acidic or basic medium. It also involves dividing the reaction into half-reactions.
- Balance each half-reaction for the elements first, then for the electrons, and finally adjust for the pH by adding H? ions in acidic medium or OH? ions in basic medium.
Each of these methods has its own place depending on the complexity of the equation and the comfort level of the user with mathematical or systematic approaches. For most educational and practical purposes, the Inspection and Algebraic methods are commonly used and provide a good balance between simplicity and rigor.
Simple Balancing Equations Worksheet
Balancing Equations Example Worksheet
Tips to Balancing Equations Worksheet
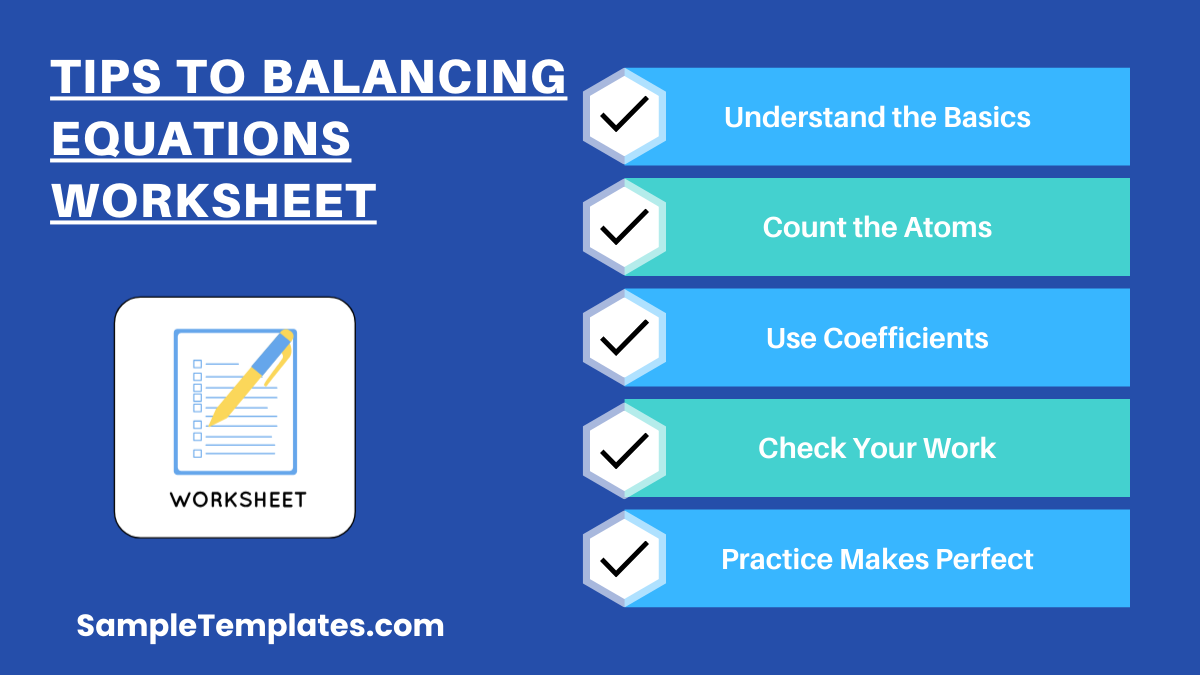
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is obeyed in chemical reactions. Here are some tips to help you effectively balance equations, especially when working on a worksheet:
- Understand the Basics: Ensure you understand the basic principles of chemical reactions, including the conservation of mass and the role of reactants and products.
- Write the Unbalanced Equation: Start by writing down the unbalanced equation from the worksheet. Make sure to include all reactants and products as given.
- Count the Atoms: List all the elements involved in the reaction and count the number of atoms of each element on both the reactant and product sides.
- Balance One Element at a Time: Begin by balancing the elements that appear in only one reactant and one product. It’s usually easier to start with the most complex molecule.
- Use Coefficients: Adjust the coefficients (numbers placed before the reactants and products) to balance the atoms. Never change the subscripts within a molecule, as this changes the actual substance.
- Balance Hydrogen and Oxygen Last: Since these elements are often found in multiple compounds and are more common, it can be easier to balance them after the others.
- Check Your Work: After you think you’ve balanced the equation, recount the atoms of each element to ensure both sides are equal.
- Consider Polyatomic Ions as Units: If a polyatomic ion (like SO?²? or NO??) appears unchanged on both sides of the equation, balance it as a unit instead of balancing each element individually.
- Practice Makes Perfect: The more you practice balancing equations, the more intuitive the process will become. Use additional worksheets or online resources to practice.
- Seek Help If Needed: If you find yourself struggling, don’t hesitate to seek help from teachers, peers, or online tutorials. Understanding why certain steps are taken in balancing equations can be just as important as practicing the skill itself.
By following these tips, you can enhance your ability to balance chemical equations accurately and efficiently, a crucial skill in studying chemistry.
Sample Balancing Equations Worksheet
Why We Say Our Templates Efficient for You
They are all worked on by experts whom we have carefully scrutinized before employing them, they all have vast experience in the field are they come in handle when you want any custom edits done on the template or when you want a completely different one. Our models are also readily available, and they come at affordable prices. You May also See Subtraction Frenzy Worksheets
For any consultations you need, contact us and speak to our customer care representatives should advise or assist you accordingly. Order your template today and reduce all the future hustles when developing your chemistry career.Impress your Chemistry Tutor by using our Balancing Equations Worksheet Template
If you have any DMCA issues on this post, please contact us!
Related Posts
Retirement Speech Samples & Templates
Weekly Schedule Samples & Templates
Contractual Agreement Samples & Templates
FREE 9+ Amazing Sample Church Bulletin Templates in PSD | PDF
Sample Business Card Templates
Sample Cashier Job Descriptions
Questionnaire Samples
FREE 10+ Sample HR Resource Templates in PDF
FREE 10+ HR Consulting Business Plan Samples in MS Word | Google Docs | Pages | PDF
FREE 49+ Sample Job Descriptions in PDF | MS Word
FREE 16+ Nonprofit Budget Samples in PDF | MS Word | Excel | Google Docs | Google Sheets | Numbers | Pages
FREE 13+ Academic Calendar Templates in Google Docs | MS Word | Pages | PDF
FREE 10+ How to Create an Executive Summary Samples in Google Docs | MS Word | Pages | PDF
FREE 23+ Sample Event Calendar Templates in PDF | MS Word | Google Docs | Apple Pages
Company Profile Samples