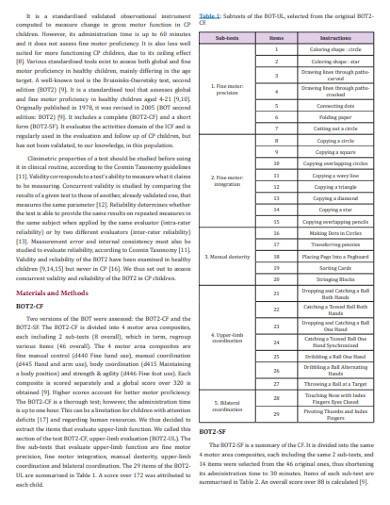
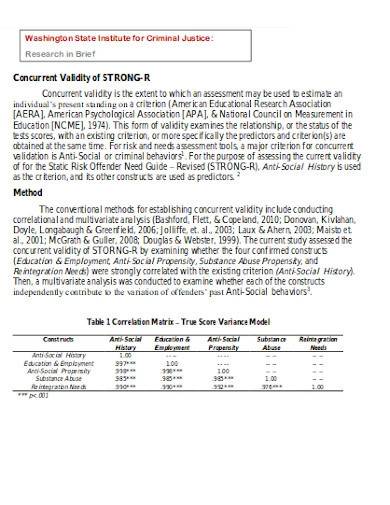
Validity covers an entire experimental concept and determines whether the achieved outcome is able to meet the requirements of a scientific research method utilized. The two types of validity include internal validity which determines how an experimental design should be structured and external validity which refers to the process of analyzing the result and determining possible causal relationships. Concurrent validity is a type of criterion validity that measures the correlation between two kinds of assessments.
FREE 10+ Concurrent Validity Samples & Templates in MS Word | PDF
1. Concurrent Validity Template
2. Factor Analysis & Concurrent Validity
3. Reliability & Concurrent Validity Template
4. Basic Concurrent Validity Template
5. Reliability and Validity Template
6. Concurrent Validity of Internet Addiction
7. Worker Concurrent Validity Template
9. Pearson Concurrent Validity Template
10. Concurrent Validity Evaluating Template
11. Sample Concurrent Validity Template
What is Concurrent Validity?
Concurrent validity presents the extent to which two different measures or assessment that are taken at the same time agrees with each other. This is a scientific method in which a researcher compares their new assessment and data analysis with one that was already tested and proven to be valid. This type of validity is a subtype of criterion validity that describes the effectiveness of the estimated performance of the examinee on an outcome measure.
How to Establish Concurrent Validity
Validity is a method used by researchers to represent the extent to which their obtained results represent reality. Research methods such as qualitative research and quantitative research are used to determine validity. Concurrent validity is established when the results of a new study or test correlate with an existing validated measure. It validates an assessment instrument by comparing its results to other tests or variables that were previously validated.
Step 1: Determine the Appropriate Method of Measurement
Make sure that your measurement and method used are high in quality and targeted to measure what you are supposed to measure. Your research study plan must be in-depth and based on a piece of existing knowledge.
Step 2: Utilize the Poper Sampling Method
Provide a clear definition of the population you are measuring to produce a valid and generalized result. Make sure that you have sufficient participants and are the appropriate representative of the population you are aiming to measure. Otherwise, it can lead to possible sampling bias as well as selection bias.
Step 3: Ensure Consistency of the Methods
Ensure that you have carefully outlined the method you will utilize in a similar way for each of your measurements. If you are interviewing or observing participants, clearly describe how certain responses or behaviors will be counted.
Step 4: Standardize the Research’s Conditions
When collecting data, make sure to keep circumstances as consistent as possible to prevent influences from external factors that could create possible variations in your results. Ensure that all participants are provided with the same information and tested under similar conditions to prevent various demand characteristics.
FAQs
What are the main kinds of validity?
The four main kinds of validity are construct validity which determines if a tool represents the thing it’s supposed to measure, content validity which assesses if the test is representing all aspects of a construct, face validity considers how suitable a content of a test is, and criterion validity which evaluates how a test can predict an outcome.
How is concurrent validity different from predictive validity?
Concurrent validity is established when test scores and criterion variables are measured simultaneously while predictive validity is established when you obtain test scores and criterion scores at different points in time.
What are the advantages of concurrent validity?
Concurrent validity provides you with a fast way to validate data and is a highly appropriate way to validate personal attributes such as IQ, strengths, and weaknesses.
Concurrent validity is a criterion validity subtype that measures how well a new test agrees with a well-established or existing test. This validity also refers to a practice of concurrently testing two different groups at the same point in time or asking groups of people to take similar tests in the same conditions. Other types of research that observers use include comparative research, documentary research, experimental research, and more.
Related Posts
-
FREE 10+ Biography Research Report Samples and Templates in PDF
-
FREE 10+ System Documentation Samples & Templates in MS Word | PDF
-
FREE 10+ Process Document Samples & Templates in MS Word | PDF
-
FREE 10+ Action Research Samples & Templates in PDF
-
FREE 10+ Longitudinal Research Samples & Templates in PDF | MS Word
-
FREE 10+ Causal Research Samples & Templates in MS Word | PDF
-
FREE 10+ Client Discovery Samples & Templates in MS Word | PDF
-
FREE 10+ Null Hypothesis Samples & Templates in MS Word | PDF
-
FREE 10+ Research Hypothesis Samples & Templates in MS Word | PDF
-
FREE 9+ Product Knowledge Samples & Templates in PDF
-
FREE 10+ Technical Documentation Samples & Templates in MS Word | PDF
-
FREE 10+ Software Documentation Samples & Templates in MS Word | PDF
-
FREE 10+ Exploratory Research Samples & Templates in PDF | MS Word
-
FREE 10+ Experimental Research Samples & Templates in MS Word | PDF
-
FREE 10+ Descriptive Research Samples & Templates in PDF