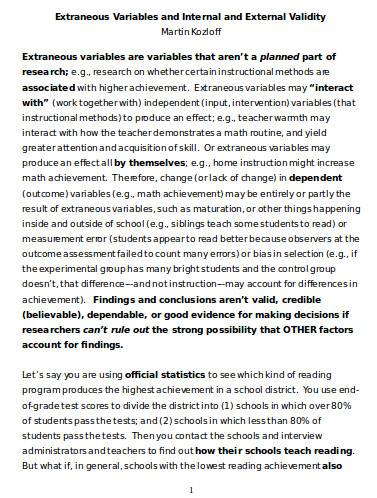
Internal validity is a critical component of any descriptive research study, as it ensures that the sample survey results are accurate and reliable. Researchers need to ensure that their study design is free from any biases or confounding variables that could affect the results. For example, a study examining the effects of a new medication list on a particular condition needs to ensure that any changes in the condition are due to the medication and not to other factors such as a change in lifestyle or other medications being taken. To maintain internal validity, researchers use a variety of techniques such as random assignment, blinding, and control groups. Random assignment helps to ensure that participants are assigned to groups in a way that is free from bias, while blinding ensures that participants and researchers are unaware of which group participants have been assigned to, thus reducing the potential for bias.
FREE 7+ Internal and External Validity Samples & Templates in MS Word | PDF
1. Internal and External Validity Template
2. Sample Internal and External Validity
3. Formal Internal and External Validity
4. Basic Internal and External Validity Template
5. Internal and External Validity
6. Internal and External Validity of Experimental Studies
7. Internal and External Validity in Clinical Research
8. Threats to Internal & External Validity
What is Internal and External Validity?
Internal and external validity are two essential concepts in research methodology. Internal validity refers to the extent to which a study accurately measures what it claims to measure, without being influenced by confounding variables. External validity, on the other hand, refers to the extent to which the findings of a study can be generalized to other populations, settings, or situations beyond the context of the original study.
How To Make Internal and External Validity?
To ensure external validity, researchers need to consider the representativeness of the sample, the ecological validity of the study, and the generalizability of the findings. Ensuring internal and external validity in research studies requires careful planning report, design, and execution. Here are some general tips on how to maximize internal and external validity:
Step 1- Use appropriate study design
The study design should be appropriate for the research question being asked. For example, if the goal mindmap is to establish causality, a randomized controlled trial may be the most appropriate design. Randomization helps to minimize the effects of confounding variables and ensure that the groups being compared are similar in all respects, except for the intervention being studied.
Step 2- Control extraneous variables
All extraneous variables that may affect the outcome should be controlled or eliminated. For example, if the study is examining the effects of a new medication on a particular condition, participants’ 7 day diet plan diet, daily exercise plan, and other medications they are taking should be controlled or eliminated.
Step 3- Participants and researchers
Blinding can help to reduce bias by ensuring that participants and researchers are unaware of which group participants have been assigned to. Appropriate statistical analysis plan can help to ensure that the results are accurate and reliable.
Step 4- Use multiple study sites
Using multiple study sites can help to increase the generalizability of the findings. Conducting follow-up studies can help to ensure that the findings are applicable over time and that they continue to have content valid.
How can you increase internal validity?
You can increase internal validity by using an appropriate study design, randomizing participants, controlling extraneous variables, blinding participants and researchers, using appropriate statistical analysis, and conducting follow-up studies.
Can a study have high internal validity but low external validity?
Yes, it is possible for a study to have high internal validity but low external validity if the study results are accurate and reliable but cannot be generalized to other populations, settings, or situations beyond the context of the original study.
Can a study have high external validity but low internal validity?
Yes, it is possible for a study to have high external validity but low internal validity if the study results can be generalized to other populations, settings, or situations beyond the context of the original study, but the study design or execution was flawed, and the results are not accurate or reliable.
In conclusion, both internal and external validity are crucial components of research methodology, as they ensure that the results are accurate, reliable, and applicable to community situational analysis. Internal validity is concerned with the accuracy of the results, while external validity is concerned with their applicability. Researchers must consider both aspects when designing and evaluating research studies to ensure that their findings are trustworthy and can be used to make informed decisions in various fields.
Related Posts
FREE 10+ Construct Validity Samples & Templates in MS Word | PDF
FREE 10+ Code of Human Research Ethics Samples & Templates in MS Word | PDF
FREE 10+ Biography Research Report Samples and Templates in PDF
FREE 10+ System Documentation Samples & Templates in MS Word | PDF
FREE 10+ Process Document Samples & Templates in MS Word | PDF
FREE 10+ Action Research Samples & Templates in PDF
FREE 10+ Longitudinal Research Samples & Templates in PDF | MS Word
FREE 10+ Causal Research Samples & Templates in MS Word | PDF
FREE 10+ Client Discovery Samples & Templates in MS Word | PDF
FREE 10+ Null Hypothesis Samples & Templates in MS Word | PDF
FREE 9+ Product Knowledge Samples & Templates in PDF
FREE 10+ Software Documentation Samples & Templates in MS Word | PDF
FREE 10+ Exploratory Research Samples & Templates in PDF | MS Word
FREE 10+ Experimental Research Samples & Templates in MS Word | PDF
FREE 10+ Descriptive Research Samples & Templates in PDF