In the realm of clinical research, precision and consistency are paramount. Our Sample Case Report Form Template stands as an exemplary tool, meticulously designed for researchers and medical professionals. Capture, analyze, and organize patient data with unprecedented ease and accuracy. As the cornerstone of reliable clinical trials, this template ensures you’re not just collecting data, but elevating the quality of your research. Dive in to streamline your documentation process today.
20+ Case Report Form Samples
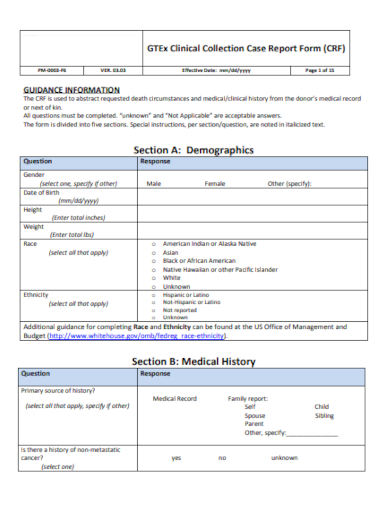
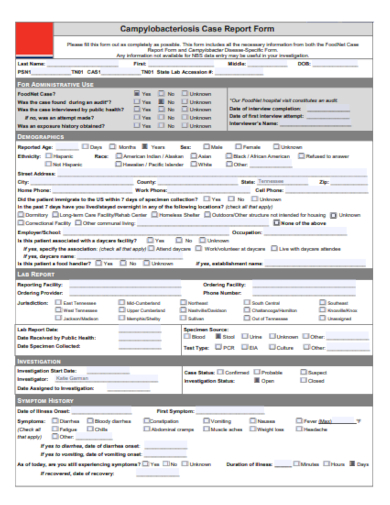
1. Case Report Form Template
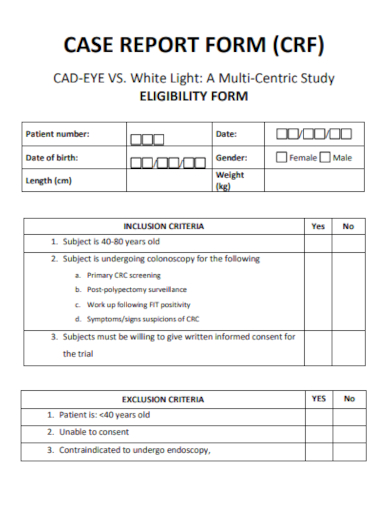
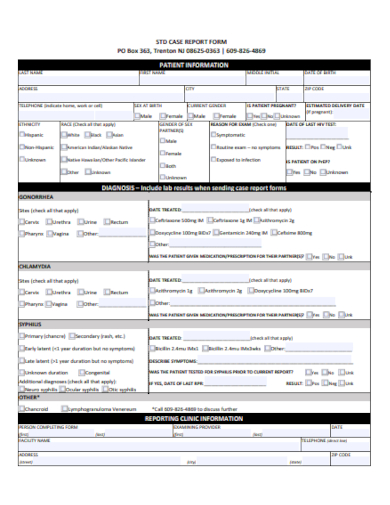
2. Clinical Case Report Form
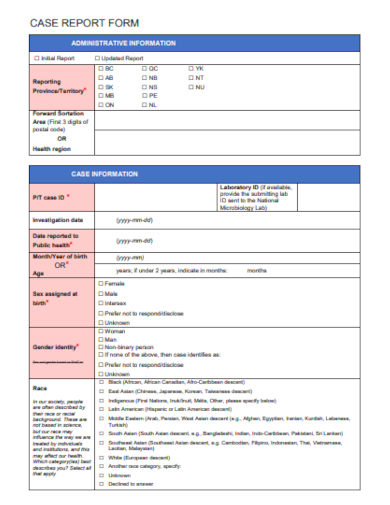
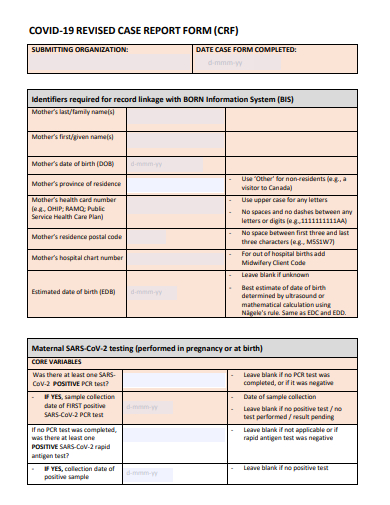
3. COVID-19 Case Report Form
4. Patient Case Report Form
5. Sample Case Report Form
6. Medical History Case Report Form
What is the Case Report Form?
The Case Report Form (CRF) is a pivotal instrument in clinical research,
designed to systematically collect specific data during a clinical trial. The primary goal of the CRF is to consolidate data from every trial participant, ensuring consistency and integrity across various sites and subjects.
Key Components of a CRF
While CRFs may vary in design depending on the study’s nature and the conducting organization, most will generally consist of:
Demographic Information: Essential details about the participant such as age, gender, ethnicity, and other relevant background data.
Clinical Data: Information directly related to the study’s objectives, like diagnostic tests, prior medical history, physical exams, and laboratory results.
Treatment Details: Specifics about the treatment or drug under study, including dosage, frequency, and any alterations to the regimen.
Adverse Events: Documenting any unforeseen medical incidents during the trial, irrespective of their relation to the treatment.
Outcome Measures: The data points the trial is particularly interested in evaluating, such as the drug or treatment’s efficacy.
The Significance of CRFs
CRFs are essential for several reasons:
They ensure Data Consistency and Quality, providing a standardized format that makes sure every researcher collects information in a uniform manner.
They help in Regulatory Compliance, ensuring that the data documentation meets the requirements of authoritative bodies like the FDA or EMA.
They Facilitate Data Analysis, with organized data making it easier to interpret and draw conclusions.
They offer Traceability, ensuring every piece of data can be traced back to its origin, vital for data auditing or verification purposes.
Electronic Case Report Forms
The advent of technology has introduced Electronic Case Report Forms (eCRFs). These are digital renditions of traditional paper CRFs and provide several advantages, including real-time data recording, fewer data entry errors, and swifter data access and sharing.
How Do I Create a Case Report Form?
Before designing a CRF, it’s essential to have a clear grasp of what you hope to achieve with your clinical trial. What are the primary and secondary endpoints? What data will be most relevant to reaching these conclusions?
Choose the Right Format
There are two main formats for CRFs: traditional paper forms and electronic forms (eCRFs). While eCRFs offer advantages in terms of real-time data entry and easier sharing, paper CRFs might still be preferred in certain contexts, especially where digital infrastructure is lacking.
Designing the Form
Keep it Simple: While it’s crucial to gather all necessary data, over-complicating the form can lead to errors in data entry.
Standardize: Ensure that the format and questions are consistent throughout, reducing variability in responses.
Clear Instructions: Each section should come with clear instructions to guide researchers or clinicians in filling out the form.
Include Essential Sections
While the specific data points you gather will depend on your study, every CRF should ideally have sections for:
Demographics
Clinical Data
Treatment Information
Adverse Events
Outcome Measures
Testing the CRF
Before rolling out the CRF for actual data collection, test it. This pilot phase will allow you to identify potential issues or areas of confusion that can be rectified before the actual trial begins.
Training the Staff
Ensure that all staff members involved in data collection are adequately trained on how to fill out the CRF. This is especially crucial for eCRFs, where staff may need training on the specific software or platform being used.
Regularly Update and Review
As the trial progresses, there might be a need to update the CRF based on new findings or challenges encountered. It’s essential to regularly review and, if necessary, modify the CRF to ensure it remains relevant and effective.
Creating a CRF is a meticulous process that demands attention to detail. It’s not just about gathering data, but doing so in a way that ensures the integrity, consistency, and relevance of the data collected. Properly designed CRFs are crucial to the success of any clinical trial, ensuring the results are valid, reliable, and ultimately contribute meaningfully to medical science.
7. Printable Case Report Form
8. Adverse Event Case Report Form
9. Clinical Study Case Report Form
10. Disease Case Report Form
11. Cancer Case Report Form
12. Generic Case Report Form
13. Electronic Case Report Form
14. Case Report Form Document
15. Police Case Report Form
16. Editable Case Report Form
17. Client Case Report Form
18. Standard Case Report Form
19. Virus Case Report Form
20. Case Study Report Form
21. Malaria Case Report Form
What Should Be in a Case Report Form?
A Case Report Form (CRF) is a structured document or tool used in clinical trials to systematically collect data from each study participant. Ensuring that the CRF is well-designed and comprehensive is crucial to the success of the trial, as it plays a pivotal role in ensuring data quality, consistency, and reliability. Let’s delve into the essential elements that should be included in a CRF:
Title and Study Information:
At the top of the CRF, there should be a title indicating the specific study or trial it pertains to. This section should also include the trial’s unique identification number, sponsor details, and protocol number.
Participant Identifier:
Each CRF must have a specific identifier for the study participant. This could be a unique code or number assigned to the participant, ensuring their anonymity and confidentiality.
Demographic Information:
This section captures the basic details about the participant, such as age or date of birth, gender, ethnicity, education level, and occupation.
Informed Consent Verification:
Before any data collection, it’s crucial to have a section verifying that the participant has given informed consent to be part of the study. This ensures ethical considerations are met.
Clinical Data:
This is the core of the CRF. It captures all relevant clinical information related to the study’s objectives, including medical history, current medications, physical examination findings, diagnostic test results, and laboratory test results.
Treatment and Intervention Details:
For clinical trials testing a specific intervention, this section captures details like drug or treatment name, dosage and frequency, route of administration (e.g., oral, intravenous), date and time of each administration, and any changes or interruptions in the treatment regimen.
Adverse Events and Side Effects:
Safety is paramount in clinical trials. This section tracks any unexpected medical occurrences or side effects that arise during the study. It should capture the description of the event or side effect, its severity and duration, action taken, and the outcome.
Outcome Measures:
This section is tailored to the specific objectives of the study. It captures the data points the study aims to measure or evaluate, providing insights into the treatment or intervention’s efficacy and impact.
Follow-up Information:
For trials that involve monitoring participants over an extended period, this section captures details about follow-up visits, including date and time of follow-up, clinical findings during the follow-up, and any changes in treatment or interventions.
End of Study Information:
This section records the details when a participant concludes their involvement in the study, including reasons for ending participation and the final status of the participant.
Comments or Notes:
A section where clinicians or researchers can add additional notes or observations that may be pertinent to the study but don’t fit into the standard categories.
A well-designed CRF is instrumental for successful clinical research. By meticulously capturing every relevant detail, it ensures that the data collected is robust, reliable, and can lead to meaningful insights in the realm of medical science.
Related Posts
Sample Sworn Affidavit Forms
Vehicle Inspection Forms Samples & Templates
Sample Employee Advance Forms
Sample Child Travel Consent Forms
Sample Testimonial Request Forms
Sample Employee Details Forms
Sample Divorce Forms
Sample Attestation Forms
Employee Performance Appraisal Form Templates
FREE 9+ Sample Presentation Evaluation Forms in MS Word
FREE 10+ School Admission Form Samples & Templates in MS Word | PDF
FREE 30+ Patient Consent Form Samples in PDF | MS Word
FREE 10+ Sample Sign Off Form Templates in PDF | MS Word
FREE 11+ Sample Medical Consultation Forms in PDF | MS Word
FREE 8+ Sample Donation Forms in PDF | MS Word