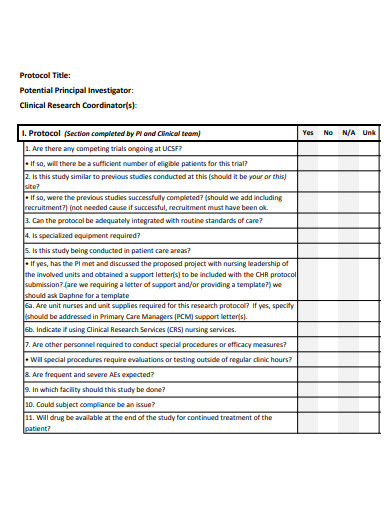
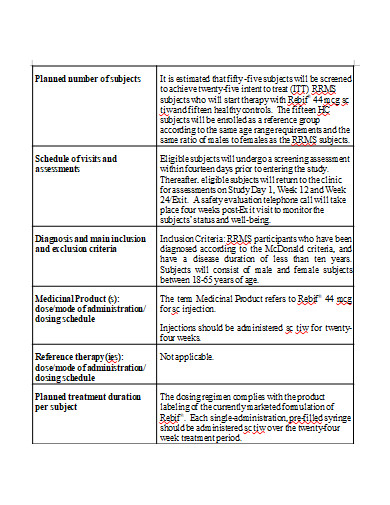
It is the process through which a group of scientists and clinicians test a new medical therapy, medicine, gadget, or method on a group of individuals to see how well it works. A clinical trial’s goal is to discover a new and better approach to treat, prevent, or cure various types of sickness. In many circumstances, the trial may be for a treatment that is not yet accessible to the rest population of persons suffering from an ailment. Special accommodations are made to allow doctors to learn more about how well a new technique works. They compare the results to the best available existing treatments in the hopes of discovering something better.
10+ Clinical Trial Samples
Clinical trials are a sort of studies to investigate novel medical interventions and assesses their impact on human health outcomes. Volunteers participate in clinical trials to test treatment options such as medications, cells, and other biological products, surgical operations, radiological procedures, equipment, behavioral treatments, and preventative care. Clinical studies are meticulously planned, reviewed, and executed, and they must be approved before they can begin. Clinical trials are open to persons of different ages, including children.
1. Clinical Trial
2. Clinical Trials Booklet
3. Clinical Trial Protocol
4. Sample Clinical Trial
5. Clinical Trial Handbook
6. Clinical Trial Overview
7. Clinical Trial Policy
8. Clinical Trial Checklist
9. Basic Clinical Trial
10. Formal Clinical Trial
11. Printable Clinical Trial
Advantages of Clinical Trial
- You might get a new treatment before it’s generally available to the general population.
- You supply researchers with data that allows them to develop better medicines.
- Because the agency that supports the study normally pays for testing and medical visits connected to the trial, your treatment costs may be reduced. Before you begin, it’s a good idea to address these charges with your medical team.
Difference of Treatment and Clinical Trial
- You may be subjected to more examinations than usual. These allow the research team to track your progress and gather data.
- You may need to discontinue or modify your present medications, as well as your diet. Always consult with your medical team before making any changes.
- In some circumstances, you won’t know whether you received the new medication or something similar (double-blind, placebo-control study). This allows the team to determine how effectively the medicine works.
- Your medical team will request documentation authorizing them to test the new medication on you (informed consent).
A trial is usually for a specific condition, and each phase may necessitate a various levels of symptoms. You may be able to take part in a trial if you meet the eligibility requirements. Certain tests may be required to establish that you are a good candidate. Your biographical information is kept private and is not linked to your name in the study.
Clinical trials do provide vital information on a treatment’s cost-effectiveness, the clinical utility of a diagnostic test, and how a medication enhances the quality of life.
Each study is carried out in accordance with a detailed strategy, or protocol. The plan specifies the sorts of patients who can participate in the trial, the schedule of tests and procedures, the medications and dosages, the necessary follow-up, and the duration of the study. It also outlines the outcomes (endpoints) to be measured and the sort of data to be collected, which we will then share with regulatory authorities to seek market authorization and with payers to obtain payment.
FAQs
What is informed consent?
The trial’s doctors and nurses will discuss the procedure to you, including its potential advantages and hazards, and then request you to sign a consent form form indicating your willingness to participate. This is your “informed consent,” and please keep in mind that signing does not bound you to the study. You have the option to exit the trial at any point during the process.
What are the phases of biomedical clinical trial?
- Phase I studies typically test new medications in a small number of people for the first time in order to determine a safe dosage range and discover negative effects.
- Treatments that were proven to be safe in phase I now require a bigger group of human volunteers to monitor for any harmful effects in phase II research.
- Phase III studies are carried out on bigger populations in various locations and nations, and are frequently the final step before a new medication is approved.
- Following country approval, Phase IV studies are conducted, and there is a need for more research in a large population over a longer term.
This could be especially beneficial if you have a major disease and have exhausted all usual therapies. These new medicines are initially tested in laboratories by scientists. They next test them on laboratory animals. Only when they believe they are safe and effective enough in these early phases can clinical trials begin on humans, first in small groups and subsequently in larger ones.
Related Posts
FREE 7+ Medical Student CV Samples in MS Word PDF
FREE 10+ Management Research Report Samples [ Waste, Wealth ...
FREE 10+ Research Data Collection Form Samples & Templates in ...
FREE 7+ Sample Product Evaluation Forms in MS Word PDF
FREE 10+ Investigation Plan Samples in PDF DOC
FREE 8+ Product Monograph Samples in PDF MS Word
FREE 22+ Endorsement Letter Samples & Templates in PDF MS ...
FREE 10+ Monograph Samples in PDF MS Word
FREE 32 White Papers in PDF
FREE 9+ Medical Records Clerk Job Description Samples in MS ...
FREE 10+ Project Risk Register Samples in PDF Excel
FREE 7+ Death Investigation Report Samples in PDF
FREE 45 SOP Templates in PDF MS Word
FREE 10+ Statistical Analysis Samples [ Methods, Data, Software ]
FREE 44+ Fact Sheet Templates in MS Word Pages | PDF